Update on DX regulations and treatment for TBC_revised
Thursday 1 February 2024
Update on tuberculosis regulations, diagnosis and treatment
Tuberculosis (TB) continues to be one of the infectious diseases with the highest mortality rates in the world, generating devastating health, social and economic consequences for the population. Every day, nearly 4,000 people die from tuberculosis and about 28,000 suffer from this preventable and curable disease. TB can affect anyone regardless of age, sex, race, social or economic status.
TB is caused by the microorganism Mycobacterium tuberculosis, or commonly known as “Koch’s bacillus”. It is transmitted when a person with active, untreated pulmonary tuberculosis coughs, sneezes or speaks, which causes aerosols to become airborne.
In immunocompetent persons, it is estimated that M. tuberculosis infection can be controlled in 90% of the cases, however, 10% of the infected population, depending on the immunological conditions, the infection can reactivate generating an active TB at some point in life, in people with HIV the risk increases from 5% to 10% depending on their immune status. TB disease usually affects the lung or the tracheobronchial tree (pulmonary TB), but can also affect other organs of the body such as the pleura, kidneys, bones, meninges, intestines, skin, genitourinary system, etc., (extrapulmonary TB).
In Colombia, TB is a public health priority. The Ministry of Health and Social Protection (MSPS) has a National Tuberculosis Prevention and Control Program (PNPCT), which coordinates sectoral, intersectoral and community actions for the diagnosis, treatment and follow-up of cases and their contacts in different settings.
In February 2020, Resolution 227 was issued, defining the technical and operational guidelines of the National TB Program, with the updating of diagnostic algorithms, treatment schemes and responsibilities of the different agents of the health system, which must include and prioritize in their territorial health plans the financial resources to meet the goals of the tuberculosis program and ensure that all public and private health care institutions guarantee that their entire population has access to the technologies for diagnosis, treatment and follow-up of tuberculosis.
The COVID-19 pandemic has jeopardized the gains made in the years-long fight to end TB and is estimated by WHO models to increase by hundreds of thousands of additional deaths per year in view of declining notifications and the difficulty people have in accessing TB diagnostic, preventive and curative treatment services as they face obstacles due to their generally vulnerable and marginalized status. It is imperative to ensure continuity of essential TB prevention, diagnosis, treatment and care services even during emergencies such as the COVID-19 pandemic, so as not to reverse what has been achieved so far.
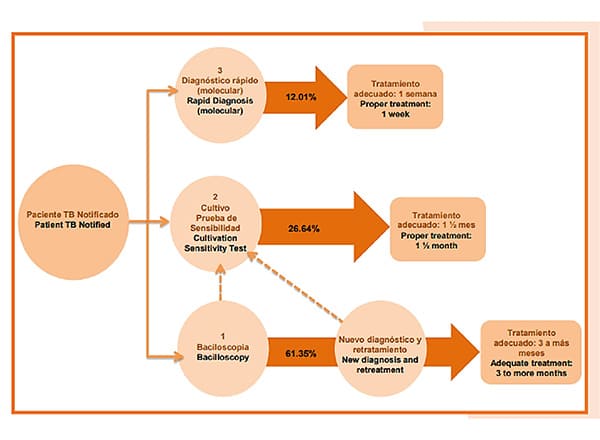
In previous years, the diagnosis of a TB case was made through smear microscopy, a solid medium culture was requested and sensitivity tests were performed in a reference laboratory, obtaining reports 10 to 14 weeks after the start of the treatment regimen. Subsequently, with the incorporation of the culture in liquid medium for sensitivity tests, the times were shortened, but not sufficiently. The technical guidelines for laboratories in the framework of Resolution 227 of 2020, establish that the methods of choice at the microbiological level are molecular techniques and liquid medium culture due to their high sensitivity, specificity and speed for the detection of cases over smear microscopy.
The use of polymerase chain reaction (PCR) is ideal for initial diagnosis as it allows results to be obtained in less than 48 hours and allows the patient to receive treatment with effective drugs from the beginning to treat TB and drug-resistant TB. In addition, it is expected that the laboratories that currently have infrastructure for the diagnosis of COVID-19 will enter to strengthen the tuberculosis network and that, by the end of 2022, Colombia will have completely migrated to the use of molecular testing to diagnose any person with signs and symptoms of tuberculosis in replacement of smear microscopy.

Within the framework of Resolution 227 of 2020, the diagnostic algorithms for active pulmonary tuberculosis were updated as follows:
1A. In people over 15 years of age who do not belong to vulnerable groups or high-risk patients presenting cough and expectoration for 15 days in general population without history of disease or immunosuppressive treatments. This algorithm includes the foreign migrant population that does not meet the criteria of algorithms 1b and 1c.
Perform: Bacilloscopy and chest x-ray. In the case of a negative result in a patient with suspected tuberculosis, always perform a liquid culture. If the smear microscopy is positive, perform molecular testing. and if positive continue with sensitivity tests to first line drugs Rifampicin and Isoniazid and follow the algorithm.
1B. In people over 15 years of age who do belong to vulnerable groups such as persons deprived of liberty (PPL), Afro-descendants, street dwellers, indigenous people, displaced persons, victims of the conflict, migrants, health workers, rural or dispersed rural population, among others. In contacts of persons affected by TB, cough and expectoration of 1 or more days of duration will be taken as criteria.
Perform: Molecular test, liquid culture and chest X-ray. In case the molecular test is positive, continue with sensitivity tests to first line drugs Rifampicin and Isoniazid and follow the algorithm.
1C. Active pulmonary TB in people living with HIV or with a history of immunosuppressive pathologies or treatments.. Indicate cough with expectoration, fever, weight loss or night sweats of any duration.
In case of suspicion of TB: Molecular test, liquid culture and chest X-ray. In case the molecular test is positive, continue with sensitivity tests to first line drugs Rifampicin and Isoniazid and follow the algorithm.
1D. Active pulmonary TB in girls or boys under 15 years of age, check for cough and expectoration for 15 days, fever greater than 8 days, weight loss or failure to gain weight in the previous three months, decreased activity level or play, and history of contact with an adult diagnosed with TB.
Take at least 2 samples of induced sputum, gastric aspirate or gastric lavage or spontaneous sputum when children are able to expectorate. Perform molecular test and culture in liquid medium to the two samples. Perform chest X-ray and tuberculin test (PPD).
The diagnostic algorithm also indicates to perform a molecular test that provides results of resistance to Rifampicin/Isoniazid in 100% of previously treated patients with risk factors for drug resistance, in addition, a complementary liquid culture should be performed when the molecular test shows negative or uninterpretable results, especially when there is a high suspicion of resistance.
In Annex 4 of Resolution 227 of 2020, there are also prophylactic treatment schedules for those who are identified as having a latent infection for TB especially for people with HIV, children under 5 years of age, people with pathologies or immunosuppressive treatments and health care workers. Prophylaxis is administered, after ruling out active TB, and a 3 or 6-month regimen is offered.
The MSPS centrally manages the purchase of medicines for the treatment of cases of susceptible, resistant and latent tuberculosis and monitors the departmental and district territorial entities for the promotion of 100% oral treatment schemes.
The treatment schemes are oriented for children, adolescents and adults of confirmed cases, which are based on the daily administration of tablets according to weight, in two treatment phases with a duration of 6 to 9 months, for the case of TB sensible.
For sensitive active TB in adults and children, the following schedule is administered: 2 months Isoniazid + Rifampicin + Pyrazinamide + Ethambutol for 56 doses Monday through Saturday, followed by 4 months with Isoniazid and Rifampicin to complete 112 doses Monday through Saturday. In people with HIV and TB without adherence or without antiretroviral therapy, it can be prolonged up to dose 196 of the second phase, or in people with osteoarticular and meningeal TB up to dose 280 of the second phase.
For people witresistent TB multidrug-resistant (MDR-TB), rifampicin-resistant (RR-TB) and extensively drug-resistant (XDR-TB), oral and injectable regimens are available .6 months with Bedaquiline, Linezolid, Clofazimine, Levofloxacin, followed by 14 months of Linezolid, Clofazimine, Levofloxacin. In children under 6 years of age, Bedaquiline is replaced by Cycloserine.
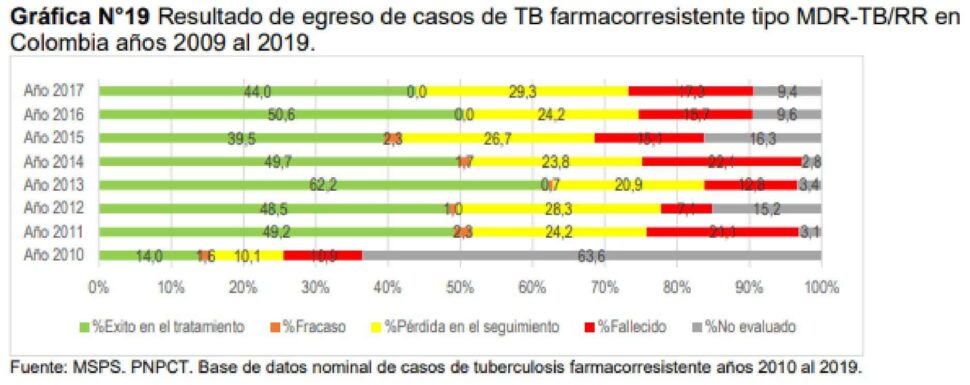
According to the report on the behavior of TB in Colombia 2016-2025, regarding the results of the treatment of cases with drug-resistant TB in the country, it is observed that in the cases catalogued MDR-TB/RR, as well as monoresistance to isoniazid and XDR-TB cases, a persistent low success in treatment compared to the sensitive TB cohort.
Treatment of TB not only stops the chain of transmission of the disease but also reduces the risk of complications or death. Therefore, the administration of TB treatment should be guaranteed during the COVID-19 contingency, encouraging adherence to treatment by facilitating follow-up administration at the virtual, home or community level, with family support.
A conservative scenario is considered in which it is estimated that a patient with inadequately treated tuberculosis can infect an average of 10 to 15 people through direct contact over the course of a year, and these in turn infect more people; 30% of those exposed become infected with TB; of those infected, 10% become ill; 50% will manifest the disease during the first two years following primary infection and the remaining 50% at some point in their lives. With the combination of this information it is possible to determine a transmission factor. If timely and adequate treatment is not provided, there is a risk that about 45% of HIV-uninfected persons with tuberculosis and, in practice, all persons with tuberculosis/HIV coinfection may die.
Therefore, based on the above, it is calculated in an average scenario that a person who is ill without treatment for one year exposes a little more than 42 people directly and indirectly to TB. Of these 12.5 will become infected (30%) and of those infected 10% will become ill (1.25 persons), 6.25 persons will become ill in the next 2 years (50%) and 6.25 may develop TB at any time in their lives (remaining 50%); for the latter TB may be more aggressive and cause severe lung lesions. For purposes of estimating costs, the factor for new cases is 1.25; with a factor of 0.625 for those who become ill in the next 2 years and a factor of 0.625 for those who become ill at some point in their lifetime. The same factor is assumed for TB and MDR TB.
Bibliography:
- Organización Panamericana de la Salud. Día Mundial de la Tuberculosis 2021 – OPS/OMS. Disponible en: https://www.paho.org/es/campanas/dia-mundial-tuberculosis-2021
- Organización Panamericana de la Salud. Tuberculosis – OPS/OMS. Disponible en: https://www.paho.org/es/temas/tuberculosis
- Ministerio de Salud y Protección Social. Resolución 227 de 2020, por la cual se adoptan los lineamientos técnicos y operativos del Programa Nacional de Prevención y Control de la Tuberculosis. Disponible en: https://www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%B3n%20No.%20227%20de%202020.pdf
- Ministerio de Salud y Protección Social. Prevención enfermedades transmisibles. ¿Qué es tuberculosis (TB)? Disponible en: https://www.minsalud.gov.co/salud/publica/PET/Paginas/Tuberculosis.aspx
- Ministerio de Salud y Protección Social. Prevención ABECÉ Tuberculosis. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/abece-tuberculosis-msps.pdf
- Instituto Nacional de Salud Colombia. Boletín epidemiológico semanal (BES). Comportamiento de la Vigilancia de Tuberculosis, Colombia, 2020. Disponible en: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2021_Boletin_epidemiologico_semana_11.pdf
- Ministerio de Salud y Protección Social. Plan Nacional de Investigación Operativa en Tuberculosis Colombia 2020-2025. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/plan-nacional-investigacion-operativa-tb.pdf
- Instituto Nacional de Salud Colombia. Boletín epidemiológico semanal (BES). Comportamiento de la Vigilancia de Tuberculosis, Colombia, semana 38 de 2020. Disponible en: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2020_Boletin_epidemiologico_semana_39.pdf
- L. Posso Arango. Costos catastróficos de la tuberculosis. Barreras para el control integral de la tuberculosis desde la salud publica en Palmira.,2019. Disponible en: https://repository.icesi.edu.co/biblioteca_digital/bitstream/10906/87252/1/TG02703.pdf
- Ministerio de Salud y Protección Social. Comportamiento de la tuberculosis y avances en la implementación del plan estratégico hacia el fin de la tuberculosis en Colombia 2016-2025. Año 2020. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/comportamiento-tuberculosis-2020.pdf
- Ministerio de Salud y Protección Social. Lineamiento técnico para la prevención y control de la TB en el marco de la contingencia generada por el COVID 19- Año 2020 https://www.minsalud.gov.co/Ministerio/Institucional/Procesos%20y%20procedimientos/GIPS23.pdf
- Organización Mundial de la Salud. GLOBAL TUBERCULOSIS REPORT 2017. Disponible en: https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf