Real-time molecular diagnostics for the diagnosis of COVID – 19
Thursday 1 February 2024
“Real-time moleculardiagnostics “
The novel rapid RT-qPCR setup for COVID diagnosis – 19
The pandemic caused by Severe Acute Respiratory Syndrome Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), was first described in China in Wuhan city (Hubei province) in December 2019 and spread to the world causing a health emergency alert. To combat the virus and support the collapse of the healthcare system in healthcare facilities, they developed rapid laboratory methods for the detection of COVID-19, implemented for workflows, which decrease the diagnostic time achieving a faster therapeutic decision and minimize the risk of contagion by operators.

Given the need to reduce the risk of possible viral spread within a population caused by the rapid transmission of SARS-CoV-2, we have sought to prevent contagion, nosocomial spread and subsequent community transmission by rapidly identifying suspected cases and predicting subsequent infectious waves of viral recurrence.
Under the principle of unification, integration and miniaturization, Sansure Biotech is able to perform lysis, nucleic acid extraction, real-time PCR amplification and fluorescence analysis in a single process with high precision. Molecular diagnosis with this methodology is one of the most important techniques that has been developed in recent years with the characteristics of nucleic acid amplification in real time. Thus when this molecular diagnosis is combined with rapid tests, it means that gene detection can conveniently achieve timely, sensitive and highly specific detection of Sars-CoV-2 virus.

Sansure Biotech with a rapid molecular diagnostic system aims to transform the traditional way of diagnosis and bring the precision of the laboratory to medicine in all health institutions and therefore to all homes. It can provide fast and accurate diagnostic results for clinical emergency, hospitalization, intensive care unit, pre-surgical, screening, follow-up, contact tracing (PRASS), public health management, travelers and other fields.
The current Gold Standard for SARS-CoV-2 detection is RT-qPCR. The standard protocol involves reverse transcription of SARS-CoV-2 RNA into complementary DNA (cDNA) strands, followed by amplification of specific regions of the cDNA. For its part Sansure Biotech by applying real-time fluorescence quantitative RT-PCR technology in the instrument, detects and quantifies the ORF 1ab region and the conserved sequence specific to the protein N gene of the coding nucleocapsid as the target regions that are designed to achieve detection in the sample through changes in the fluorescent signal.
This system automatically performs rapid extraction, purification and amplification of nucleic acids in approximately 40-50 minutes in a special single-test or 4-test package, providing fast and reliable results for the diagnosis of coronavirus and additionally of other pathogens and specific molecular markers.
The samples validated in this methodology are nasopharyngeal or oropharyngeal swab, Bronchial and Bronchoalveolar lavage, Tracheal Aspirate, Sputum, and Saliva, which are deposited in Sansure’s exclusive viral transport medium which allows the viability and storage of the samples in both refrigeration and freezing, it also provides its own inactivating medium whose purpose is the loss of infectious capacity of the virus contained in the sample, thus avoiding large infrastructures for the use of the medical device.
The analyst can give the printed result or transmit it to a PC via an LIS communication system. The equipment is equipped with sophisticated software capable of processing the fluorescent signals and automatically classifying the results as positive or negative without the need for operator intervention.
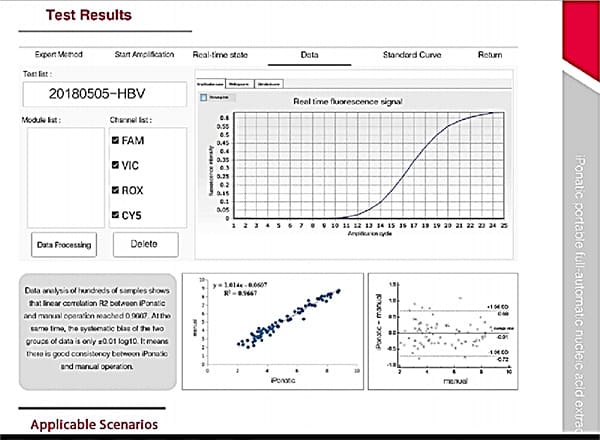
It has excellent hardware and software design to ensure repeatability and accuracy of amplification data with the following features:
- Correlation coefficient: 0.980 – ~1.000
- Repeatability: CV <5.0%.
- Sensitivity: ≥95%.
- Specificity: ≥95%.
- Detection limit: 200 copies/mL – 2 copies/10µL
Additionally, in response to the recently updated multiple variants of the new coronavirus, Sansure Biotech bioinformaticians reanalyzed the alignment of detection targets demonstrating that their kits detect all reported variants.
Annar Health TechnologiesSansure Biotech, together with Sansure Biotech as a strategic ally, is committed to biotechnological advances in diagnostic methodologies that provide low complexity laboratories with state-of-the-art technology, offering your institution the best for sample processing for the detection of COVID-19 and with the appropriate support to achieve the best results, always thinking of supporting the medical area with agile, truthful and quality information.
Bibliography
- Corman, V. M., Landt, O., Kaiser, M., Molenkamp, R., Meijer, A., Chu, D. K. W., … Drosten, C. (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.
Eurosurveillance
,
25
(3), 1-8. https://doi.org/10.2807/1560- 7917.ES.2020.25.3.2000045 - Sequencing and Genomic Analysis Unit – Directorate of Public Health Research – National Institute of Health. Tests for the molecular detection of SARS-CoV-2 by RT-PCR used in Colombia. National Institute of Health, 2020.
- http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1794-24702020000300043
- https://hrcak.srce.hr/index.php?show=clanak&id_clanak_jezik=355586
- https://www.akralab.es/producto/solucion-pcr-covid19-poc/
- http://eng.sansure.com.cn/index.php?g=portal&m=article&a=index&id=82
- http://ncbi.nlm.nih.gov/pmc/articles/PMC8425784/
- https://www.eurosurveillance.org/docserver/fulltext/eurosurveillance/25/3/eurosurv-25-3-5.pdf?expires=1633014090&id=id&accname=guest&checksum=81F6356B4ED449CD399358E056AC27EE



